FDA clears RedDrop’s blood-collection device
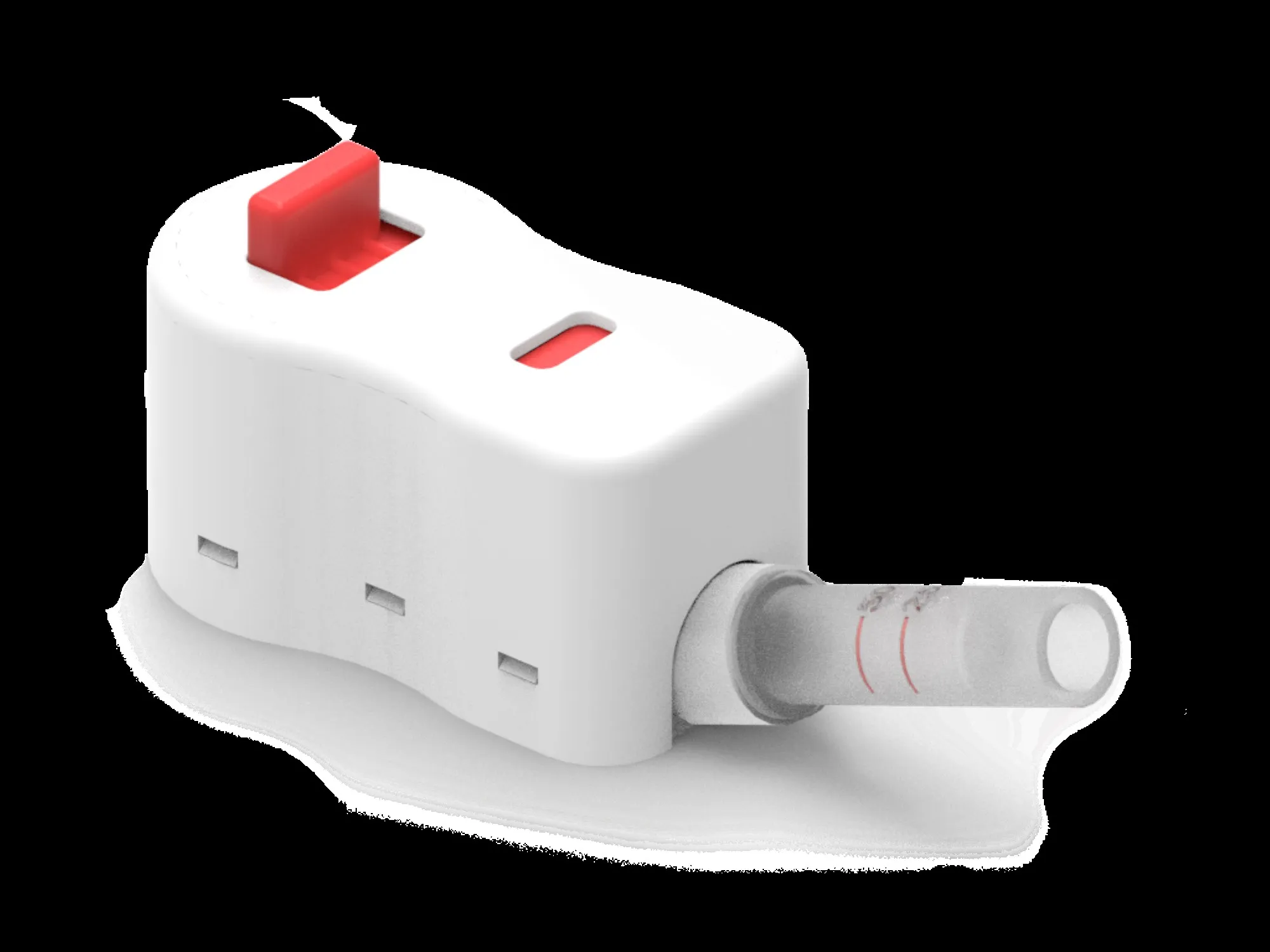
FORT COLLINS — RedDrop Dx Inc., a Fort Collins-based client and portfolio company of Innosphere Ventures, has received clearance from the U.S. Food and Drug Administration for its blood-collection device, RedDrop One.
According to the company, the device offers several advantages over traditional blood-collection methods, including the ability to collect a larger volume of blood more easily with increased reliability and reduced discomfort for the user. The device is designed to meet the needs of both patients and health-care providers by offering a more efficient and less painful blood-collection experience.
The device’s effectiveness was supported by a clinical trial with 100…
THIS ARTICLE IS FOR SUBSCRIBERS ONLY
Continue reading for less than $3 per week!
Get a month of award-winning local business news, trends and insights
Access award-winning content today!





