Less invasive: Boulder docs test new spinal treatment

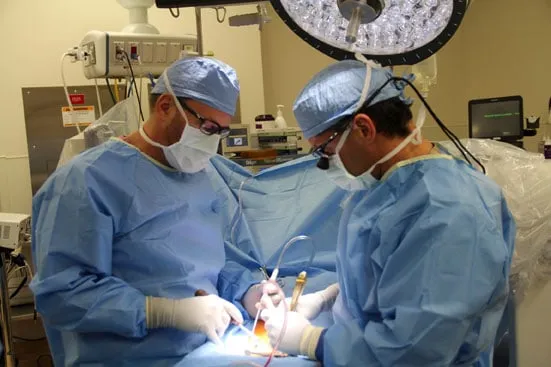
BOULDER — Boulder Community Health, in partnership with Boulder Neurosurgical and Spine Associates, is the only location in Colorado participating in a clinical trial to evaluate a new treatment for a spinal condition called degenerative spondylolisthesis. The trial is controlled by the U.S. Food and Drug Administration; surgeries for qualified patients are performed at Boulder Community Health by the surgeons of Boulder Neurosurgical and Spine Associates.
[caption id="attachment_229287"…THIS ARTICLE IS FOR SUBSCRIBERS ONLY
Continue reading for less than $3 per week!
Get a month of award-winning local business news, trends and insights
Access award-winning content today!
